Sodimate feed systems are widely used with sodium carbonate:
– To remove hardness from water caused by calcium and magnesium minerals. Hard water causes a variety of problems for treatment facilities and distribution lines, such as lime scaling or mineral buildup, which can lead to clogging and equipment damage. Many people also report hard water to have an unpleasant taste. Sodium bicarbonate, also commonly called soda ash, is added in excess to the water and these chemical reactions occur:
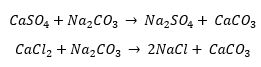
The soda ash allows the precipitation of the calcium trapped in the calcium carbonate which can then be removed through filtration or sedimentation.
– To remove the aggressiveness from water caused by a too high concentration of carbon dioxide. Aggressive water can cause rapid wear of pipes and a release of potentially toxic metal elements from the pipes. To prevent this problem, soda ash is added to the water. It increases the pH and captures the carbon dioxide. Below are the chemical reactions.
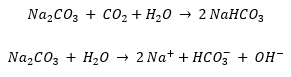
Sodimate soda ash feed systems are typically installed in municipal water treatment facilities. A complete feed system for soda ash handling typically includes a storage container, such as a big bag or hopper, a feeding unit and a mounted slurry system integrating a slurry tank, skid pumps and controls.






