The main differences between hydrated lime and quicklime are their reactivity & their chemical composition. Hydrated lime and quicklime are both calcium compounds. In its hydrated state, calcium is called calcium hydroxide, and in its pure state it is called calcium oxide, or quicklime.
Calcium oxide has a heavy density (65lb/ft³) and is more reactive than hydrated lime.
To simplify, hydrated lime is the result of adding water to powdered quicklime, putting it in a kiln or oven, and then pulverizing it with water. The resulting lime has a density of about 35lb/ft³, and is called calcium hydroxide.
It is necessary for calcium oxide (quicklime) to be slaked in a controlled environment because it can create heat that reaches up to 120 degrees Fahrenheit. Calcium hydroxide, or hydrated lime, is already neutralized, so it will not undergo oxidation and can be mixed with water in our system, for water ph control, lime slurry addition, lime slurry mixes, soil rehabilitation and more.
How do you determine whether to use hydrated lime or quicklime?
If dry, the process feed rate determines the choice between using hydrated or quicklime. Just remember that quicklime is more “reactive” than hydrated lime. However, in some processes, hydrated lime is not suitable even if we inject it dry, and vice versa with quicklime.
The best example is flue gas treatment, also known as Flue Gas Desulphurization, used in coal fire plants, cement industries, glass industries, and incinerators to reduce their HCl, Sox and Nox emissions. Some of these systems require hydrated lime to filter or catalyze the particles emitted after combustion, while other systems use quicklime. The principles are exactly the same, and the feed rates will be quite similar. However, the process and chemistry of the flue gas dictates when to use quicklime or hydrated lime.
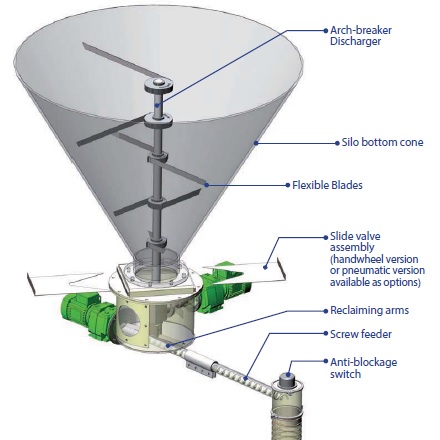
If the system requires a lime slurry, the main way to determine if hydrated lime would be more suitable than quicklime is to know the amount that a given process requires. In systems that demand large quantities of lime, quicklime would be the preferred material as the density is twice the density of hydrated lime, which reduces the storage and transportation costs. However, quicklime’s hydrophobic reaction with water requires a lime slaker to be used in the process. The quick lime is generally received in pebbles of about one quarter to one eighth of an inch, or in powder form(<300µ). The slaking of the pebble lime and powdered quick lime has to be engineered in respect to their exothermic reactions. The lime slaker mixes quicklime with water to create calcium hydroxide in a solution which is called lime slurry. Slakers are good for a high volume consumption, or high demand, of calcium. However, when a smaller or medium lime solution is requested, hydrated lime is more efficient because the equipment required to use the hydrated lime is simpler and does not need to be designed to handle an exothermic reaction. In this case, the powder is fed with screw conveyors directly into the slurry tank equipped with a slurry mixer, and water is added to match the requested lime slurry concentration (%).
What equipment is mainly used with hydrated lime?
The volume of lime you need will determine what container capacity is necessary. The main containers used are silos, hoppers, and big bags. Discharging mechanisms include Sodimate mechanical bin activators, air injection systems or simple vibrators pads. The lime can be fed and conveyed by different means, including rotary air locks, screw feeders, conveyor belts, drag chain conveyors or pneumatic conveyors. If the lime has to be put into lime slurry, a lime slurry tank can be requested and equipped with a slurry agitator and a water supply control valve.
To transport the lime slurry solution we use pumps. We generally recommend peristaltic pumps or progressive cavity pumps, or any type of lime slurry pumps our customer feels confident using.
The pumps needed with quicklime are exactly the same as with a hydrated lime solutions. However if the lime is injected into a solution, we will also need a lime slaker. The lime slaker is generally fit with a mixer and agitator, all made of stainless steel, in addition to a vacuum system (lime scrubber) that washes down and evacuates the heat and steam created from the reaction between the calcium oxide and water. Besides that, quicklime works exactly the same as hydrated lime, including storage, metering, and conveying.
What kind of system is recommended for a feed rate of 100 lbs /hr of lime?
If this is for water treatment, we would recommend the use of hydrated lime because the feed rate is low. Depending on the budget, the lime could be stored within silos, hoppers, or even big bags or super sacs. The use of volumetric screw conveyors to feed and convey is likely the best & most cost effective solution of lime feeders, while in use with a lime silo.
FAQs
Quicklime, also known as calcium oxide (CaO), is a versatile chemical compound that is commonly used in many different industries. It is produced by heating limestone in a kiln, which causes the calcium carbonate to break down and release carbon dioxide, leaving behind calcium oxide. Quicklime is highly reactive and can be used for a variety of applications, such as soil stabilization, water treatment, and steel production. It can also be used as a flux in glass production and as a component in cement. Additionally, quicklime has several industrial uses, including as a desiccant and as a raw material for the production of calcium hydroxide (slaked lime) and other chemicals.
When quicklime is added to water, a chemical reaction occurs that releases heat and forms calcium hydroxide, also known as slaked lime. This reaction is highly exothermic, meaning it releases a large amount of heat. The chemical equation for this reaction is CaO + H2O → Ca(OH)2. The resulting calcium hydroxide is a white, powdery substance that is commonly used in construction, agriculture, and other industries. This reaction is also known as the slaking of lime, and it is an important process for making lime mortar and other lime-based products.
When quicklime is heated with silica, a chemical reaction occurs that results in the formation of calcium silicate, also known as slag. The reaction equation is CaO + SiO2 → CaSiO3. This reaction is commonly used in metallurgy to extract metals from their ores, as the slag produced can separate from the metal and be easily removed. Calcium silicate is also used as an insulating material in high-temperature applications, such as in furnaces and boilers. The reaction between quicklime and silica is highly exothermic, meaning it releases a large amount of heat. It is important to handle quicklime with care, as it can cause severe burns if it comes into contact with the skin or eyes.